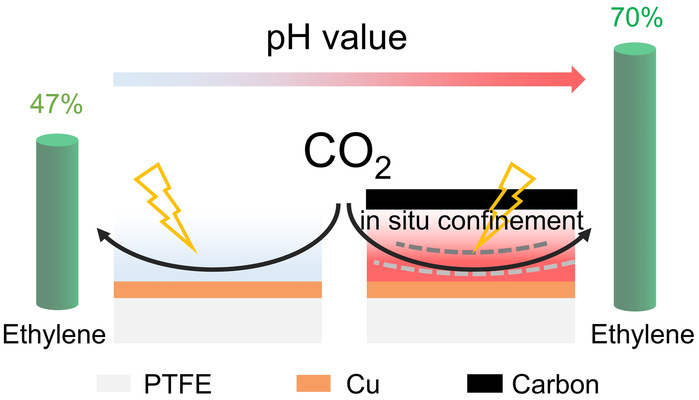
Electrocatalytic CO2 reduction reaction (CO2RR) is one of the most promising routes to facilitating carbon neutrality. An alkaline electrolyte is typically needed to promote the production of valuable multi-carbon molecules (such as ethylene). However, the reaction between CO2 and OH- consumes a significant quantity of CO2/alkali and causes the rapid decay of CO2RR selectivity and stability. Here, we design a catalyst-electrolyte interface with an effective electrostatic confinement of in situ generated OH- to improve ethylene electrosynthesis from CO2 in neutral medium. In situ Raman measurements indicate the direct correlation between ethylene selectivity and the intensities of surface Cu-CO and Cu-OH species, suggesting the promoted C-C coupling with the surface enrichment of OH-. Thus, we report a CO2-to-ethylene Faradaic efficiency (FE) of 70% and a partial current density of 350 mA cm-2 at -0.89 V vs. the reversible hydrogen electrode. Furthermore, the system demonstrated a 50-hour stable operation at 300 mA cm-2 with an average ethylene FE of ~68%. This study offers a universal strategy to tune the reaction micro-environment and a significantly improved ethylene FE of 64.5% was obtained even in acidic electrolytes (pH=2).
https://pubs.acs.org/doi/10.1021/jacs.2c13384