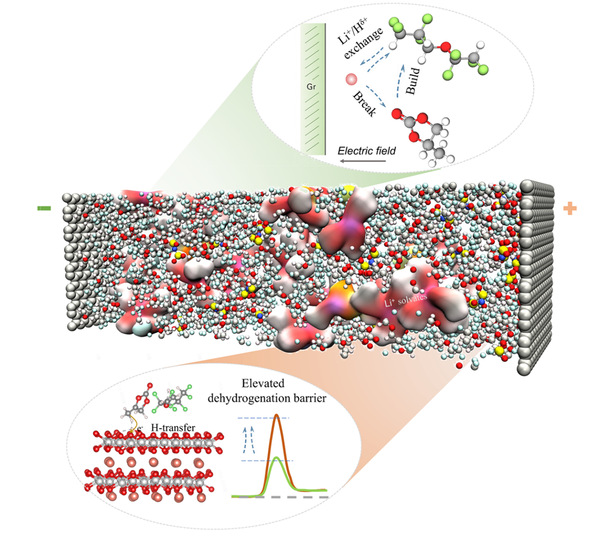
The electric double layer (EDL) plays a pivotal role in governing interfacial composition and electrode behavior in electrochemical systems. However, the intricate relationship between EDL architectures and electrochemical processes remains elusive. Here we elucidate the fundamental significance of hydrogen bond polarity within the EDL in orchestrating the interfacial lithium-ions (Li+) exchange dynamics. At charged interfaces, the electropositive aprotic hydrogen (Hδ+) and Li+ ions exhibit comparable electrostatic responses, resulting in their competitive effect for the solvent oxygen sites, which modulates the de-solvation process and ultimately impacts battery performance. Based on this, we propose a solvent-centered de-solvation mechanism, wherein the microenvironment with enhanced hydrogen polarity in the EDL facilitates solvent displacement from Li+ coordination shells. Furthermore, the presence of polar hydrogen at charged cathode interface can effectively anchor uncoordinated solvents molecules, increasing the energy barrier for detrimental dehydrogenation reaction. As a result, electrolytes design based on this strategy enables remarkable electrochemical stability, achieving over 2000 cycles in a 5 V dual-ion battery. In addition, the Gr||NCM811 pouch cell exhibits exceptional longevity, retaining 90.2% of its initial capacity after 1,000 cycles.
DOI: 10.1002/adma.202509735